Extending shelf life through validated statistical modelling
Assigning an appropriate shelf life is one of the most data-driven and scrutinised steps in pharmaceutical product development. While stability testing generates the evidence base, the real challenge lies in extracting meaningful conclusions — particularly when data variability, time constraints, or product complexity limit conventional interpretation.
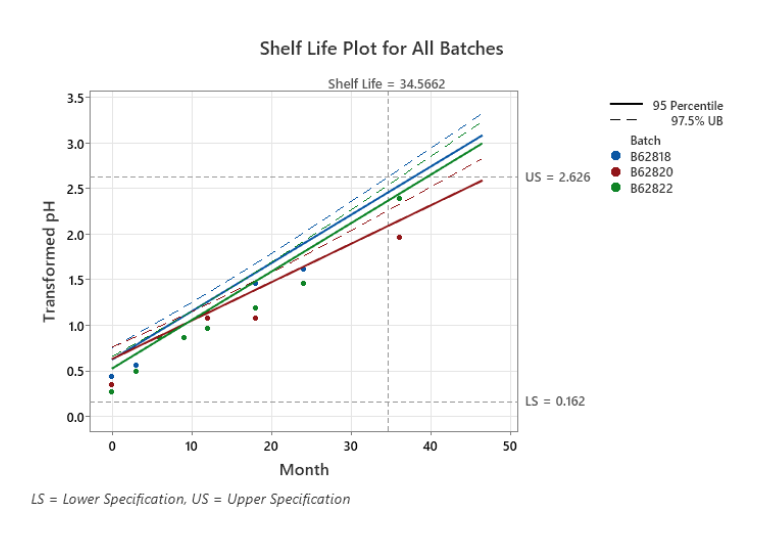
At Jaychem, we have implemented a validated statistical modelling platform to support stability data analysis in accordance with ICH Q1E – Evaluation of Stability Data. This allows our scientists to quantitatively assess degradation trends, evaluate model fit, and predict product behaviour beyond observed time points using statistically justified regression techniques.
By applying this approach, Jaychem can scientifically bridge the gap between “pass” and “fail” results to identify the maximum justifiable shelf life consistent with product quality, safety, and efficacy.
A Data-Driven, Compliant Approach
Our statistical software and procedures are:
Validated in accordance with GAMP5 and internal QA standards
Aligned with regulatory expectations
Supported by clear data traceability and audit trails for every analysis
All outputs are reviewed and interpreted by cross-functional teams across Quality, Regulatory Affairs, and Manufacturing, ensuring consistency between the scientific rationale and the regulatory submission narrative.
Regulatory Recognition
Following a series of technical assessments, Jaychem’s approach has received formal regulatory acceptance, confirming the robustness of our statistical methodology. This validation reinforces confidence in our data-driven decisions and provides a reproducible framework for future submissions.
Delivering Real Value for Customers
Extending shelf life is not merely an analytical exercise — it delivers tangible benefits:
Improved supply chain flexibility, enabling more efficient production planning
Reduced product wastage through longer expiry dating
Enhanced product availability, ensuring critical medicines remain accessible when needed most
Commitment to Continuous Improvement
Jaychem continues to invest in data analytics, statistical validation, and regulatory science capability. Our goal is to integrate advanced quantitative methods into every stage of product lifecycle management — from stability studies to post-approval change evaluations — so that our customers gain both scientific confidence and commercial advantage.